The solute must be relatively insoluble in the solvent at room temperature but much more soluble in the solvent at higher temperature. Solvent for recrystallization will depend on the state that an element is in.
Here S s View the full answer.

. The chosen recrystallization solvent will dissolve the compound when hot but not at room temperature. The second solvent will not dissolve the compound at any temperature. Although in theory there always should be a good single solvent to perform a recrystallization the question is also if it is readily available and reasonably.
The solvent should dissolve soluble impurities well at room temperature. There are several factors to consider when choosing a solvent for recrystallization of a solid compound. 2 List ten solvents used mostly for recrystallization.
The second solvent solvent 2 should induce crystallization when added to a saturated solution of your compound in the primary solvent. Procedure for Determining a Recrystallization Solvent Place about 50 mg of the sample in a test tube. Add about 05 mL of cold solvent.
Select all that apply a A solute that is at equilibrium with undissolved solvent. The recrystallization solvent should NOT dissolve the substance to be purified at room temperature but it should dissolve it well at the solvents boiling point 2. Add a small quantity of appropriate solvent to an impure solid.
2 List ten solvents used mostly for recrystallization. If the sample dissolves completely the solubility in the cold solvent is too high to be a good recrystallization solvent. Four solvents are tested to see if they are appropriate solvents for recrystallization.
An educated guess can save some time. This testing can be accomplished by putting a small amount of your solute about the. Water is the best solvent to crystallize acetanilide because water is the cheapest solvent and its solubility in water at 100C is ten times its solubility at 25C.
Ethanol Petroleum ether acetone and water were tested. C A solution contains the. So dissolve acetanilide in boiling water and cool the solution to room temperature when acetanilide crystallizes out.
You want a solvent to have high solubility at high temperature plus you want to have low solubility of the solute at low temperature. Moreover all impurities in a mixture should be either highly dissoluble in the cool solvent or practically insoluble in the solvent at all temperatures. Both alcohols tend to fulfill these criteria with regard to recrystallization of many organic species.
If the element or compound is solid NaCl would work a solvent. The relationship of solute and solvent can be best described as like dissolves like. 475 7460 Views.
Lets take a look at the details of the recrystallization process. Cool the solution to crystallize the product. What advantages des water have as a recrystallization.
What is Kf for this solvent. The essential characteristic of your suitable solvent is that the compound chosen for purification must be significantly more soluble in the hot solvent than in the cold solvent. If the solvent is not specified you will need to test a variety of solvents to determine what will work best for the solute you are trying to recrystallize.
For a two-solvent recrystallization you should have one solvent solvent 1. 25 Votes Characteristics of a Good Recrystallization Solvent. Summary of Recrystallization Steps.
975 which is highly desirable. When selecting a suitable solvent for recrystallization chemists work to the general rule like dissolves like when considering the polarity of the chemical to be recrystallized and the polarity of the solvent. Click to see full answer.
For a two-solvent recrystallization you should have one solvent solvent 1 in which your desired compound is soluble at the boiling point. In general solvents for recrystallization are classified in. The normal means of purification of a compound are by recrystallization.
The amount of solvent required is relatively small which saves costs. Moreover in an ideal world youd like the solubility of any impurities to remain higher than the target compound ie theyll stay dissolved while your target crystallizes out. Provide in the table physical properties for these solvents that help choosing the best solvent.
Apply heat to dissolve the solid. Usually however the structure of a compound may not be known so the solvent must be chosen by carrying out solubility tests. For a good recrystallization solvent you want good solubility in hot solvent and poor or insoluble in cold solvent and if the impurities are either insoluble in hot solvent you filter them off or soluble in cold solvent that is a plus.
The first recrystallization solvent will dissolve the compound at all temperatures. Choosing the proper solvent is very important in recrystallization because it will help us to increase our yield. The majority of the purified sample is recovered here.
B A solvent that is at equilibrium with solute. 1 How would you pick the good solvent for the recrystallization of your chemical. A solution of 02113 g of water dissolved in 250 g of a solvent freezes at 115 C below the freezing point of the solvent.
The first part of this experiment involves carrying out solubility tests on known compounds. A finding a solvent with a high temperature coefficient. The criteria used to choose an appropriate recrystallization solvent includes.
Recrystallization works only when the proper solvent is used. Ideally which things never are a solute should be quite soluble in hot solvent and poorly soluble in the cold solvent so that on cooling the bulk of the solute crystallizes out. Use vacuum filtration to isolate and dry the purified solid.
At the same time impurities that are present must either be soluble in the solvent at room temperature or insoluble in the solvent at a high temperature. Why is ethanol a good solvent for recrystallization. The solid must generally have a significantly greater solubility at higher temperatures and the solubility should decrease rapidly when the temperature is lowered.
If we will use inappropriate solvent the recrystallization. The compound when choosing a recrystallization solvent.
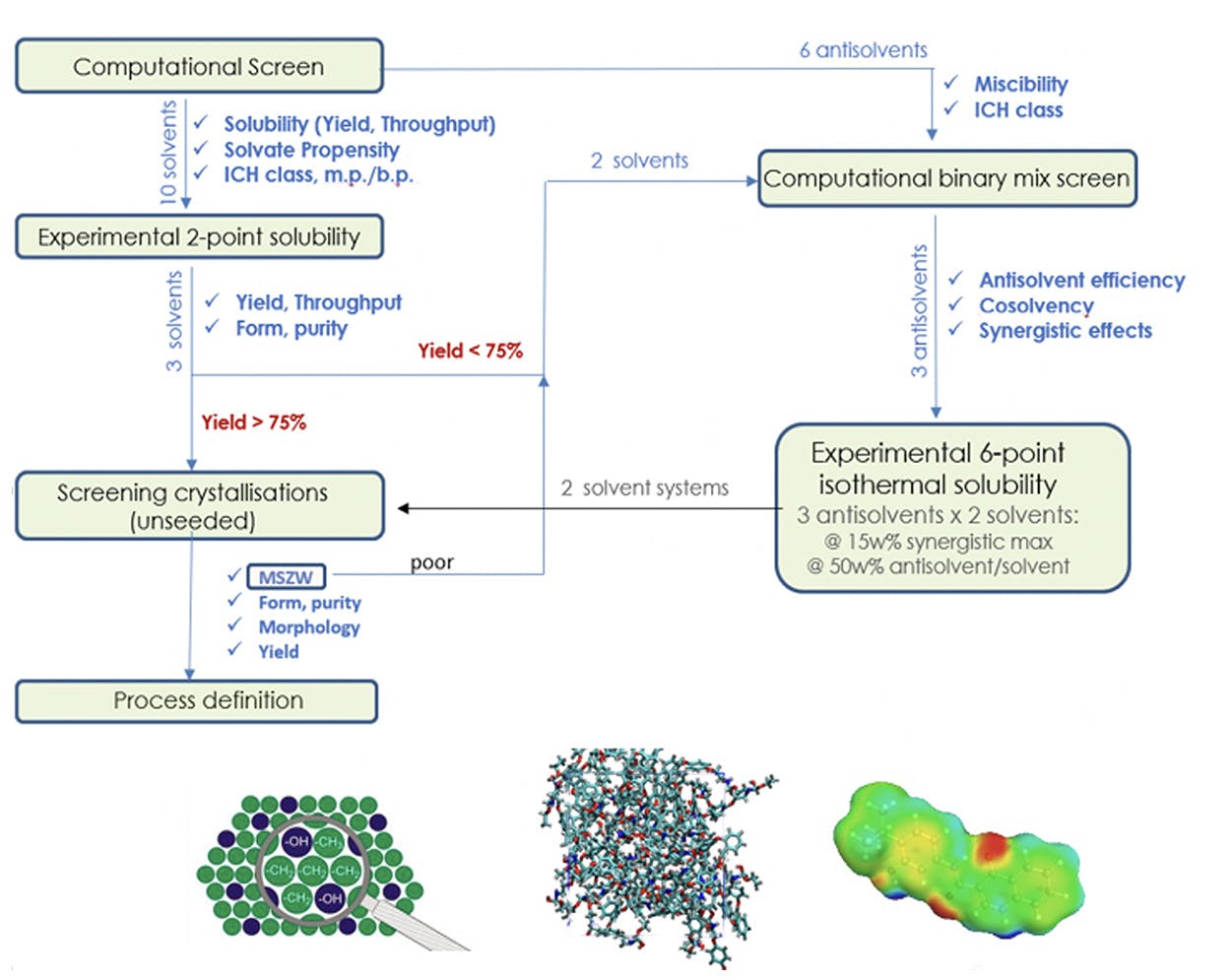
Webinar How Do You Choose A Solvent And Design Your Crystallization Faster Apc
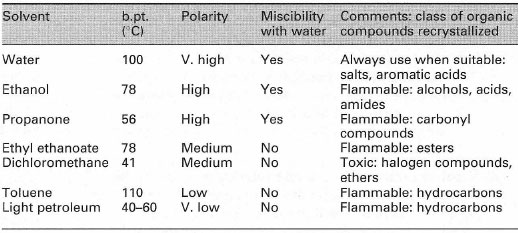
Recrystallization Laboratory Techniques

Homework Best Solvent For Crystallization Mass Recovered From Crystallization Purity Of The Product Chemistry Stack Exchange

0 Comments